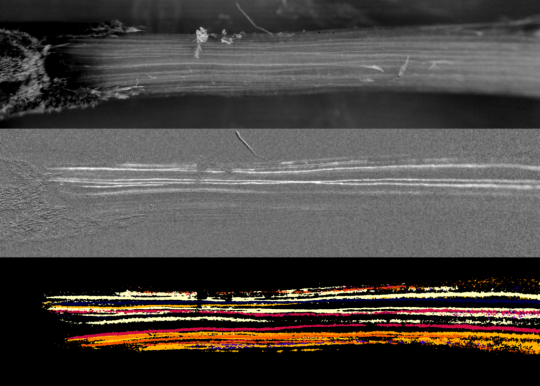
The optical method, as the name suggests, is a technique that relies heavily on optics to identify the visible changes that accompany drought stress in plants. These changes occur because light interacts differently with xylem that is water-filled vs air-filled, resulting in a subtle change in brightness that can be captured using a camera or document scanner. See the overview for more details.
To accurately assess xylem vulnerability it is very important that the major cavitation events in the tissue sample can be seen clearly. If a large proportion of the xylem cavitation cannot be clearly visualized then the possibility of error in measuring the real xylem vulnerability becomes greater. This is certainly evident for leaf veins, where the midrib is often more vulnerable than the minor veins, causing a potential underestimate of vulnerability if, for example, midrib cavitations are less well resolved than minor vein cavitations.
A good signal from the sample is critical to getting clear and distinct images of xylem embolism events. This depends on three key components: the signal to noise ratio coming from the light sensor – see Overview: A Note on Noise for more details, and the anatomy of the sample and the illumination, which are covered below.
Anatomy
The interconnected conduits of the xylem are not on the surface of plants, as would be ideal for the technique, but centrally embedded and surrounded by several layers of cells. The type of cells, the number and thickness of layers, and the number, size and arrangement of xylem conduits, all affect the way light is reflected and absorbed. Consequently a signal from a small bundle of xylem conduits, sparsely embedded amongst many layers of fibrous cells, will be much weaker than a big bundle of large-diameter xylem, under a single layer of non-fibrous cells.
Given all this it seems quite remarkable that it’s possible to detect any signal at all in some plants, however it’s worth remembering that plants have evolved to maximise light penetration, to ensure an even distribution of light energy among the layers of photosynthetic cells. The optical properties of the cells that make up the palisade, bundle-sheath, and bundle-sheath extensions, have all been shown to help channel light into the leaf. Bundle-sheath extensions in particular, which are layers of cells that extend from the xylem and phloem (the vascular bundle) to the upper and lower epidermis of the leaves, are in most heterobaric plants (plants with bundle-sheath extensions) completely transparent, providing an excellent window to the xylem underneath.
Leaves of heterobaric species and grasses are very simple to measure due to their optical properties, but stems, thicker leaves/midribs/petioles require a different approach, and some of the layers of cells must be carefully removed to expose the vasculature, thereby maximising the penetration of light.
For stems this is relatively easy, and simply requires carefully peeling off the bark and phloem. This can usually be done with a needle, scalpel or finger nail, although care should be taken to ensure that any damage to the xylem is avoided or minimised. Removing material from midribs or petioles to view the xylem can be more challenging, but is best achieved using a dissecting microscope, a steady hand, and a sharp surgical blade or razor blade. Taking sections of a neighbouring leaf beforehand can help to identify the amount of material to remove without touching the xylem. The addition of transparent gels can significantly enhance the surface transmission of light, but these must be used with caution to ensure that the substance is inert (acoustic gels are usually good), and that bubbles do not form as the gel dries.
Illumination
The influence of the sample anatomy on the light available for detection by the sensor is important, but ultimately dependant on the initial input of light provided by the illumination. It is therefore important that the illumination be bright, unobstructed, and appropriately positioned to maximise light exposure.
For opaque stems and petioles reflected light will provide the clearest image. For leaves, transmitted light generally provides the clearest image, although where the leaf/midrib is thick and layers have to be removed, it may be better to use reflected light.
Summary
- Illumination and light sensor sensitivity are key to getting good visualisation of embolism events
- Plant anatomy influences light penetration and signal strength
- Plants with bundle-sheath extensions and fewer layers of cells above and below the vascular bundle provide the best imaging
- For other plants layers of cells should be removed using a dissecting microscope and sharp blade
- For stems, the bark and phloem should be removed to expose the xylem
- Transmitted light is (generally) best for leaves
- Reflected light is best for stems